

COOLING TECHNIQUE: Liquid, integrated
COIL: Figure-8 stimulation coil, liquid cooled
COIL POSITIONING: One-hand operation w/snap lock
INTERFACE: Touch
MAPPING: Caps, MT-assistance on board
PROTOCOLS: FDA-cleared* protocols pre-installed
RESOURCES: Membership access with tutorials, instructions, marketing materials
HEAD POSITIONING: Integrated HeadNest™
COMFORT: AIR MASSAGE
FABRIC: Durable Nubuck Leather
COLORS: 6 colors available
HEIGHT: Electric height adjustment with “Coil follows movement” function
LEG REST: Electric
CONTROLS: Pushbutton Panels
Give your TMS system a personal touch. Adapt the appearance of your Blossom TMS™ Chair to your needs. Choose from a variety of colors and materials to match your clinic's aesthetic. Enhance patient comfort and satisfaction with a chair tailored to your specifications.
The Blossom TMS Therapy system is engineered for clinical efficiency. Built from the ground up for clinical use, it offers reliable and consistent performance, allowing for seamless back-to-back treatment sessions.
The Blossom TMS Therapy System is indicated for the treatment of Major Depressive Disorder in adult patients, who have failed to achieve satisfactory improvements from prior antidepressant medication.*
The patients like it a lot and feel more comfortable with this. I am really glad that I bought the Blossom machine.
The system is affordable with the best value for the money I have seen so far.
Blossom TMS has transformed my practice. It's so easy and quiet to use. Nothing should "go over your head" except TMS.
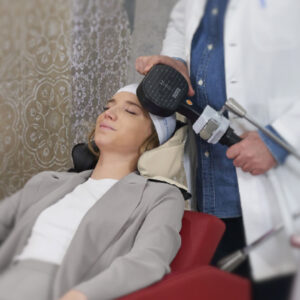 HeadNest™ is an easy way to secure the patients head during the therapy session.
HeadNest™ is an easy way to secure the patients head during the therapy session.
The patent-pending HeadNest™ reduces head movement during stimulation sessions and makes TMS easy. It reduces the risk of not keeping the coil exactly at the selected stimulation location during the entire treatment time.
HeadNest™ is very comfortable although it allows a very stable head placement. It can be easily adjusted to the individual head shape using the integrated pump. This makes treatment preparation a very easy task. It also supports the neck so that the treatment session is even more comfortable for the patient.
HeadNest™ is removable so each patient can have their own personal HeadNest™. It can maintain a stable shape over a long period of time. This allows the preparation to be accelerated even further.
HeadNest™ comes as standard with our Blossom TMS Chair.
FEATURES:

Unique features such as AIR MASSAGE make the session as comfortable as possible for the patient.
The integrated coil holder can be easily adjusted to the desired position and reliably holds the coil at the stimulation site.
With “Coil follows movement” function: If the height of the chair is changed, the coil automatically follows the change.
As standard, the chair has a comfortable leg rest and electric height adjustment on board.
INCLUDING:
AVAILABLE IN:
Fango, Camel, Inca Brown, Bordeaux, Blue Periwinkle, Vintage Gray

Phone: 833.3BUY.TMS (+1.833.3289.867)
Email: Sales@sebersmedical.com
Address: 230 S Broad Street, 17th Floor Philadelphia, PA 19102
Regulations in the United States
*United States Federal Law regulates the sale of Medical Devices. The Blossom TMS Therapy System is indicated for the treatment of Major Depressive Disorder in adult patients, who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode. (K220625) All other devices on this website are not approved and/or cleared for use in treatment and/or diagnosis in the United States. All investigational devices must be labeled in accordance with the labeling requirements of the IDE regulation (§ 812.5) and bear a label that states:
“CAUTION.
Investigational Device. Restricted by federal (or United States) law to research use.”
© 2024 SEBERS MEDICAL