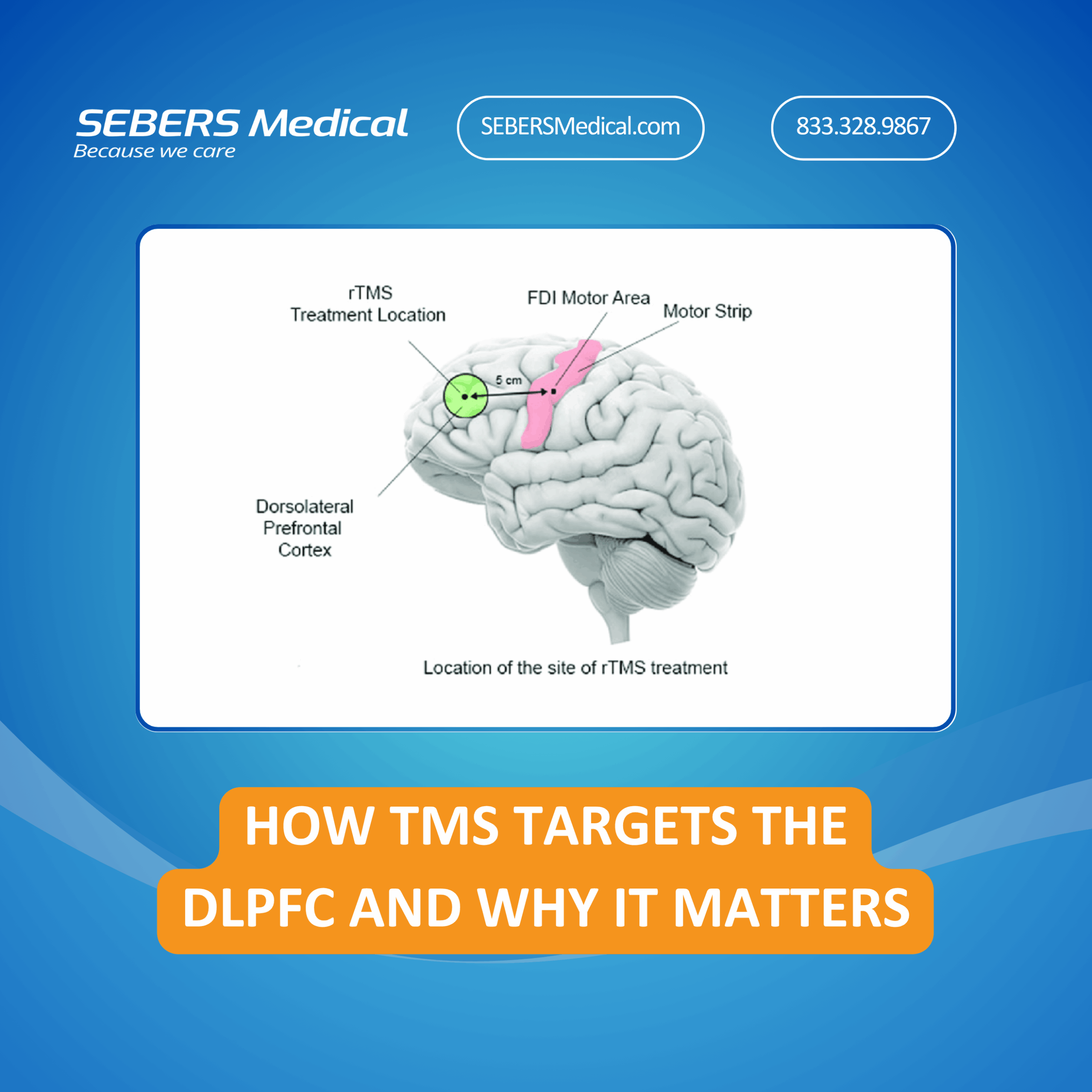
Transcranial Magnetic Stimulation (TMS) is more than just a buzzword in modern psychiatry—it’s a scientifically backed, FDA-cleared treatment that offers new hope for people with treatment-resistant depression. But while the therapy itself may seem cutting-edge, understanding why it works can bring powerful insight to both patients and providers. One of the most vital components of...




