When it comes to Transcranial Magnetic Stimulation (TMS), the technology behind the treatment is as critical as the therapy itself. At SEBERS Medical, we’ve taken every aspect of TMS therapy into consideration when designing the Blossom TMS device, ensuring it not only meets but exceeds the expectations of clinicians and patients alike. Here are three standout features that set the Blossom TMS device apart from the competition.
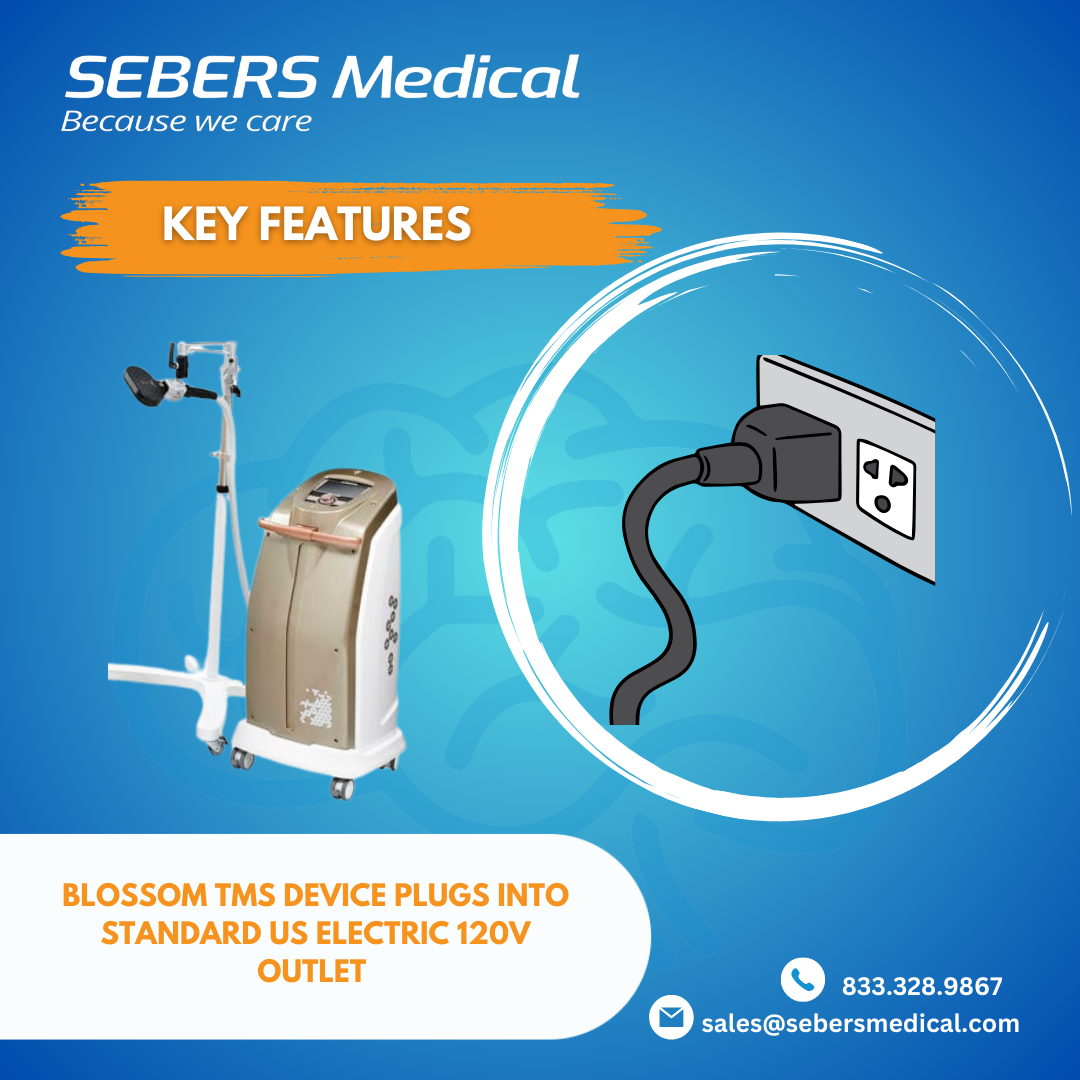
1. Plug-and-Play Simplicity: Powered by Standard 120-Volt Outlets
One of the most significant advantages of the Blossom TMS device is its compatibility with standard 120-volt outlets. This feature simplifies installation and setup, making it incredibly easy for clinics to incorporate TMS therapy without needing expensive electrical modifications. Whether you’re in a large medical facility or a smaller outpatient clinic, the Blossom TMS device can be powered up and ready to go with the simple plug-and-play convenience.
2. Shhhh: Significantly Quieter Operation
Several TMS devices rely on air cooling, which necessitates a hollow coil to allow airflow. While they can be effective, this design has a significant downside: it can be noisy. The Blossom TMS device, however, utilizes an innovative oil-cooling system that effectively suppresses this noise. The oil within the coil acts as a sound barrier, resulting in a much quieter operation. This quieter performance not only enhances patient comfort but also creates a more serene environment for both patients and clinicians.
3. Cool and Consistent: Maintaining Optimal Temperature with Oil Cooling
Running cool is essential for any TMS device. The Blossom TMS device’s oil-cooling system isn’t just about reducing noise; it also ensures the coil remains at an optimal temperature throughout treatment. Our oil-cooled design maintains a consistent, cool temperature, promoting better performance and longevity. This feature not only safeguards the device’s internal components but also enhances the overall safety and effectiveness of each treatment session.
The Blossom TMS device is a testament to the power of thoughtful design and engineering. With its ability to plug into standard outlets, operate quietly, and maintain a cool temperature during use, it’s clear that our focus is on delivering a superior experience for both clinicians and patients. These three key features highlight the innovation behind the Blossom TMS device, making it an ideal choice for any TMS provider looking to offer the best in patient care and clinical outcomes.
About SEBERS Medical
SEBERS Medical is a leading manufacturer of innovative medical devices, specializing in the production of the Blossom TMS device. Our mission is to advance mental health treatment through cutting-edge technology and exceptional quality. With a dedicated team of experts and a commitment to excellence, we strive to make a positive impact on the lives of those we serve.
Contact Us: If you have any questions or would like more information about our products, please don’t hesitate to contact us. We are here to help!
Stay connected with us for more updates and news by following our blog at https://sebersmedical.com/news/